Reclaim Patient–Centered Care with Simple, Yet Smart, Medical Practice Technology
Deliver phenomenal patient and physician experiences with an integrated EHR, Practice Management, and Revenue Cycle Management (RCM) platform from OmniMD. We integrate EHR, Practice Management, and Revenue Cycle Software and Service into one solution to let you spend more time with patients, increase productivity, ensure information exchange, and improve your bottom line.

OUR FEATURES

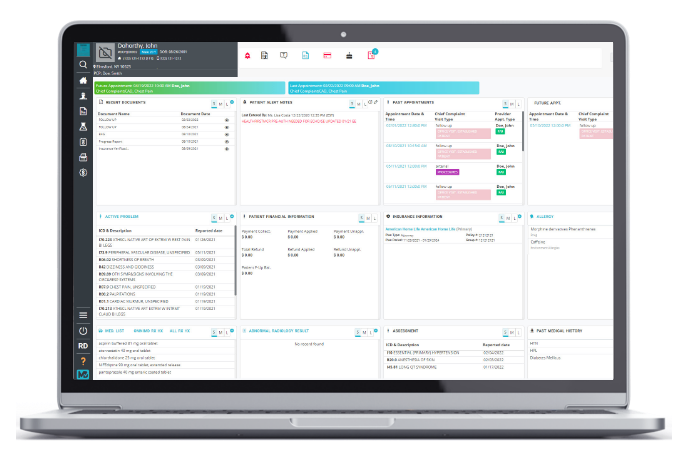
EHR Software
Simple yet savvy patient charting, decision-making, data transfer, and reporting.

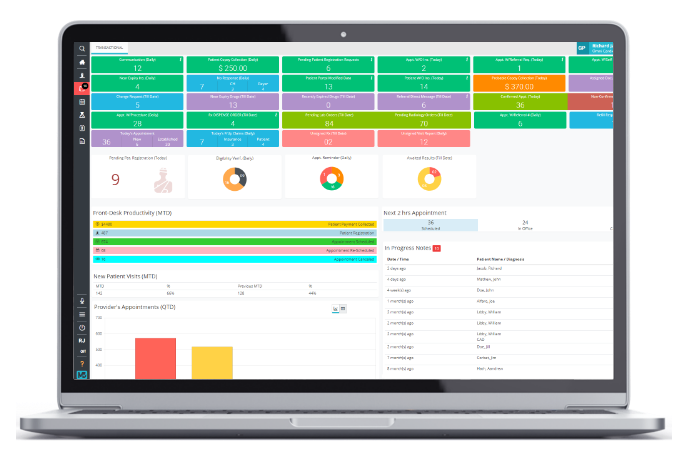
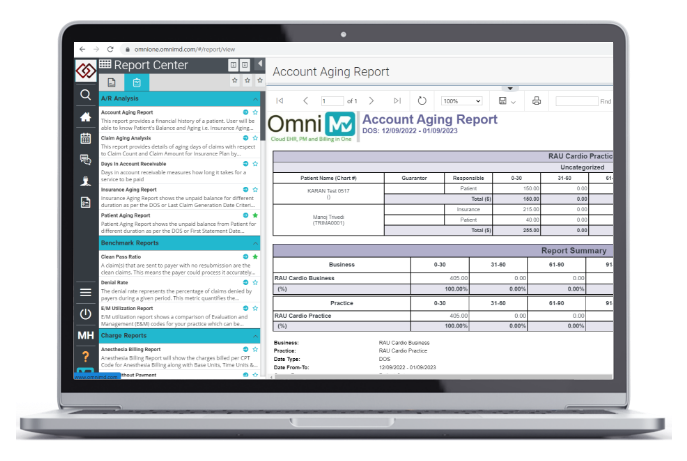
Practice Management
Integrated practice management and billing software for better scheduling.

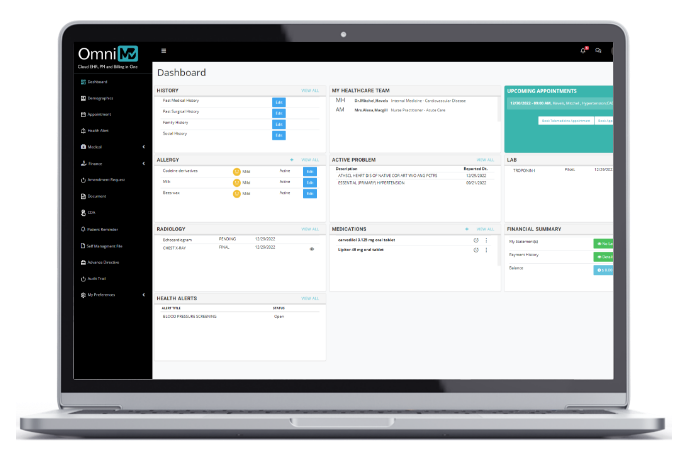
Patient Portal
Engage your patients with a wide variety of self-help tools and time-saving features.

Telehealth
Enable virtual visits to reduce travel and improve the patient experience.

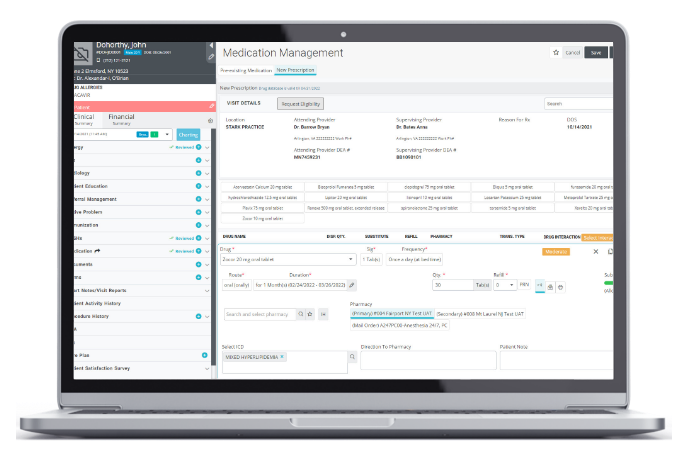
ePrescribing
Fast, safe, and securely prescribed controlled and non-controlled drugs.

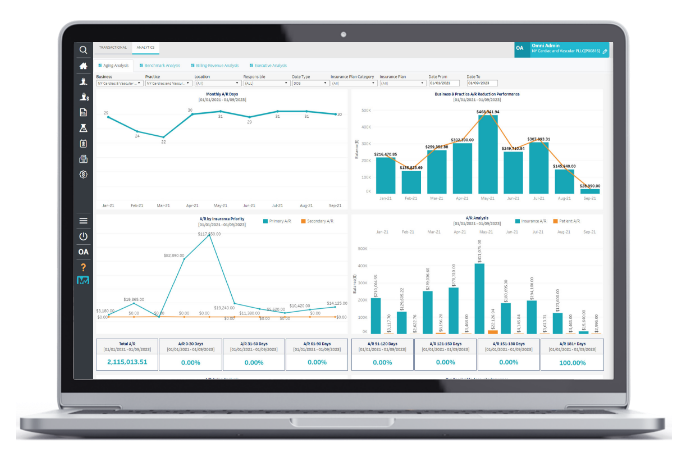
Managed Billing Services
Reduce staff and office footprint with outsourced revenue cycle management.
WHY CHOOSE OMNIMD
%
Healthcare Professionals
%
Healthcare Facilities
%
Customer Service Excellence
%
Patients Records
%
Uptime
%